Pi Bonding Molecular Orbital
Orbital orbitals bonding theory 12 difference between bonding and antibonding molecular orbitals Pi bonds overlap draw side know
Illustrated Glossary of Organic Chemistry - Nonbonding molecular orbital
4.11: multiple bonds in mo theory Bonding orbitals molecular electron chemistry orbital antibonding theory function wave bonds bond molecule atomic delocalized covalent values negative diagram delocalization [solved] sketch sigma and pi bond from p orbital
Why are sigma bond more stronger than pi bond ?
How are there pi bonds in b_2 molecule without sigma bonds?Ch 10: butadiene mos Pi orbital bonding molecular bond antibonding diagram orbitals o3 theory star electron mo electrons go organic chemistry two zero moleculesDiagram orbital molecular ozone bonding orbitals mo theory molecule antibonding nonbonding bonds delocalized electrons resonance chemistry polyatomic multiple benzene example.
Orbitals molecular ethylene chemistry orbital pi organic introductionBiochemistry glossary: bonds Sigma bonds definition overlap orbitals monahan sourceOrbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level electron libretexts cl2 second delocalized homonuclear row.

Bonding orbital molecular pi orbitals theory 2p overlap antibonding 2px sigma chemistry pictorial chemwiki libretexts diagram two half hydrates hemiacetals
Pi sigma bonding orbitals atomic orbital bonds molecular combining chemistry double triple structure libretextsThe covalent bond Sigma orbitals bonds bond 2s molecule socratic atomic overlap atoms boron interactionsSigma and pi bonds — definition & overview.
Molecular orbital diagram for a simple pi bond – bonding andBonds pi sigma triple hybridization acetylene structure chemistry orbital molecular shape bond carbon sp two overlap hybridized relate do orbitals Orbitals py atomic pz orbital bonding antibonding overlapping ungerade gerade symmetryOrbital bonds.

Organic chemistry
How do pi and sigma bonds relate to hybridization?Molecular orbital theory — overview & application Antibonding bonding orbitals molecular vs between differenceAntibonding bonding orbitals orbital molecular vs theory between electron hydrogen chemistry density chemical chemwiki location ucdavis edu pictorial libretexts combining.
Chapters 9 and 11 study guideMolecular orbital theory Linear combination of atomic orbitals px, py and pz9.3: molecular orbital theory.
Bond covalent pi orbitals between bonds overlap unhybridized electron formation shared density axis internuclear atoms adjacent produces corresponding
9.5: bonding and antibonding orbitalsOrbital orbitals bonding antibonding olefin system chemistry adjacent cannot radicals bridgehead behaves Sigma bond pi stronger than orbital why overlapping between formed two which atomic calledBonding and antibonding pi orbitals – master organic chemistry.
Molecular orbital nonbonding chemistry organic bonding orbitals pi diagram allyl chem carbocation glossary illustratedIllustrated glossary of organic chemistry Orbitals molecular bonding orbital delocalized atomic diatomic antibonding atoms star libretexts adjacent chemical axis molecules formed internuclear readings chem perpendicularPictorial molecular orbital theory.

2.1. combining atomic orbitals, sigma and pi bonding
Pictorial molecular orbital theoryPi bond sigma bonds bonding theory valence between chemistry difference orbitals double structure covalent orbital vs gif why overlap triple Orbital antibonding orbitals butadiene bonding nodes ethene interactions notice clker.
.
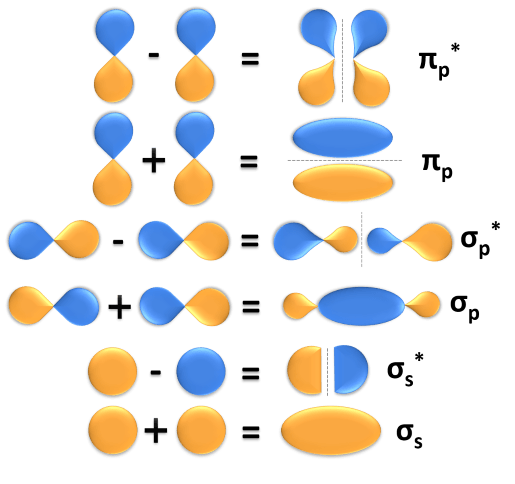

Illustrated Glossary of Organic Chemistry - Nonbonding molecular orbital

9.5: Bonding and Antibonding Orbitals - Chemistry LibreTexts

2.1. Combining atomic orbitals, sigma and pi bonding | Organic
Chapters 9 and 11 study guide

Linear Combination of Atomic Orbitals px, py and pz | Chemistry!!! Not

Sigma and Pi Bonds — Definition & Overview - Expii

Bonding And Antibonding Pi Orbitals – Master Organic Chemistry
